
Global ginsenosides research achievements timeline
- 1962-1965: The Shibata Laboratory of Natural Medicinal Materials in Tokyo, Japan, has proposed for the first time, the chemical structures of panaxa-diol and -triol.
- 1966: Shoji Shibata published the production methodology of 3-o (2β-D-glucopyranosyl-β-D-glucopyranosyl) 20(S)-protopanaxadiol.
- 1968-1984: Odashima,S. (Japan) reported that ginsenoside Rh2 inhibits proliferation of hepatoma (liver cancer) cells.
- 1987: Yun, T.K. published the anticancer effects of the ginsenosides.
- 1991: Ota, T (Korea) published the metabolic pathways of the ginsenosides.
- 1991: Kikuchi,Y. (Japan) reported that ginsenoside Rh2, when combined with the chemotherapy drug cisplatin, exert synergistic antitumor effects.
- 1993: Tode,T. published that ginsenoside Rh2 inhibits proliferation of human ovarian cancer cells.
- 1994: Kikuchi,Y. (Japan) published the metabolic pathways of orally-administered ginsenosides in the body.
- 1996: Kitagawa,I. published that ginsenosides inhibit invasion and metastasis of tumor cells.
- 1998: Akao,T. and Kobashi,K. discovered that compound K (CK) is the main anticancer metabolite of protopanaxadiol-type ginseng saponins synthesized in the body.
- 2000: Mainland China developed ginsenoside Rg3 to be the first-class anticancer drug.
- 2002: Taiwan;s first generation ginsenosides health food products completed animal experiments and clinical trials.
- 2006: The ginsenoside Rh2 product, developed by a Chinese pharmaceutical company, was put on the market.
- 2016: The rare ginsenoside product Redsenol Capsules containing 16 rare ginsenosides was developed in Canada.
- 2017: The rare ginsenoside product Redsenol-DAG Sublingual Pills containing 8 highly active rare ginsenosides was developed in Canada.
Research Breakthrough
The scientific community discovered the biological properties of rare ginsenosides about 30 years ago. In recent years, continued in-depth studies proved that rare ginsenosides play a positive role in enhancing health and improving life quality.

Research dilemma #1
Research dilemma #2
Research dilemma #3
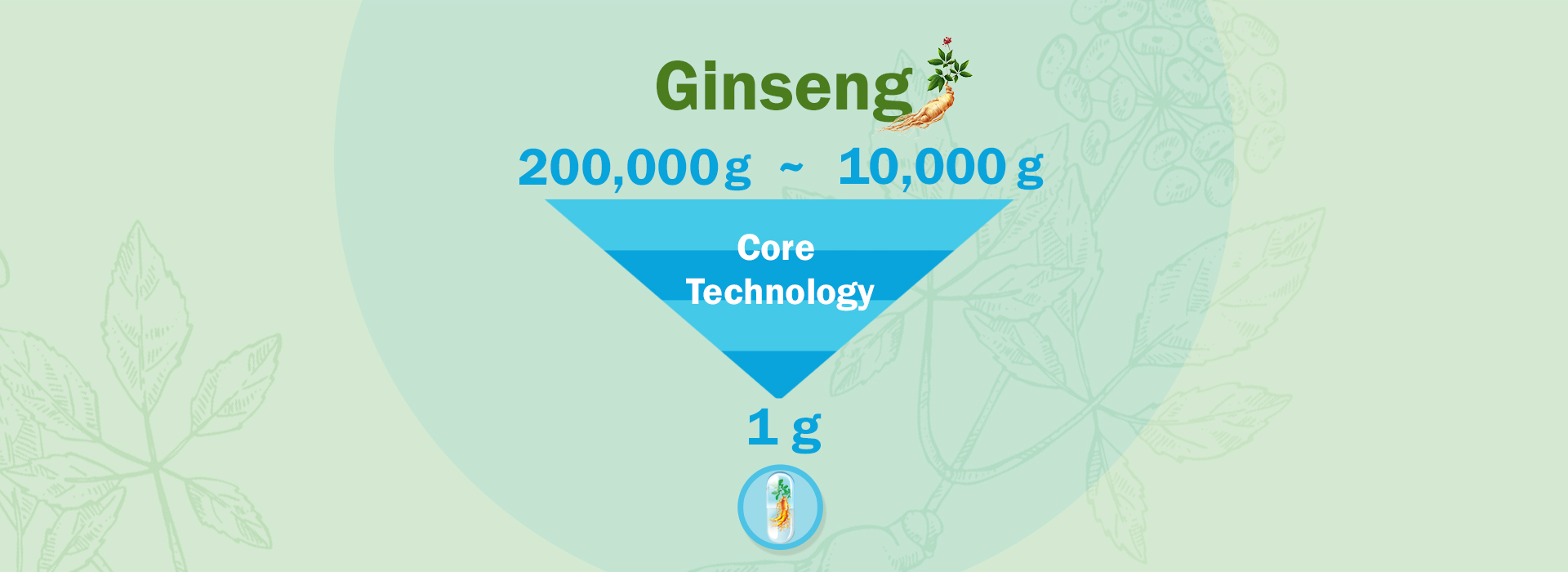
Four core technologies owned by
Canada Royal Enoch
Canada Royal Enoch Phytomedicine Ltd.,engaged in phytomedicine research for over 10 years,eventually conquered the difficulties in extraction, processing, transformation, and formulation of ginseng extracts and invented 4 core technologies.Our GMP standard manufacturing workshop, located in Vancouver, Canada, is capable of industrialized production. Our products have already benefited users from North America and Europe.
Raw material processing technology
By employing unique steam explosion technology, a new type of American red ginseng, which contains various prototype ginsenosides and rare ginsenosides, could be successfully obtained.
Ginsenosides extraction technology
By employing new molecular structure directional modification technology, prototype ginsenosides and rare ginsenosides are separately extracted.
In vitro biotransformation technology
By employing unique highly directional sugar degradation and dehydration transformation technology, prototype ginsenosides could be successfully transformed into rare ginsenosides.
Drug preparation technology
By employing nano solid dispersions and microcapsule technology, multiple rare ginsenosides are formulated into water-soluble Redsenol capsules and Redsenol-DAG sublingual pills. They act together synergistically to achieve an augmented effect.
